
| Parasite | Enteromyxum leei |
|---|---|
| Taxonomy | Myxozoa, Myxosporea, Bivalvulida |
| Hosts | More than 40 marine fishes including tiger puffer (Takifugu rubripes), Japanese flounder (Paralichthys olivaceus), red seabream (Pagrus major), spotted knifejaw (Oplegnathus punctatus), gilthead seabream (Sparus aurata), Mediterranean rainbow wrasse (Coris julis), blenids and ocean sunfish (Mola mola). |
| Disease name | Myxosporean emaciation disease |
| Infection site | Digestive tract |
| Clinical signs | Diseased fish exhibits characteristic severe emaciation including sunken eyes and bony ridges on the head (Fig. 1). Mucous liquid in the digestive tract and a flimsy intestinal wall are evident. |
| Parasitology | Many parasites are observed in the intestinal epithelia and lumen. Most of them are multinucleate developmental stages (10-20 Êmin diameter) (Fig. 2). In particular, the parasite hardly sporulates in tiger puffer. A spore is equipped with 2 obliquely arranged polar capsules (length 15.9-19.1 (average 17.5) Êm; width 7.9-11.0 (9.2)) Êm whose size is different between host species (Fig. 3). The parasite has a broad host range (over 40 marine fishes around the world) (Padros et al., 2001). Since this parasite transmits from fish to fish (Diamant, 1997; Yasuda et al., 2002), its infection spreads rapidly in fish farms. |
| Pathology | Proliferation and sloughing of the intestinal epithelia are observed (Fig. 4). As a result, the osmoregulatory and nutrition system may be impaired, causing the emaciation of a host. |
| Health hazard | Since this parasite is not infectious to human, it is harmless in food hygiene. |
| Diagnosis | Observe Diff-Quik stained imprints of the intestinal wall. However, technical expertise is required to diagnose based on the morphology of development stages. Leptotheca fugu (Fig. 5) and Enteromyxum fugu (Fig. 6) also parasitize to the intestine of tiger puffer. The former is highly pathogenic and the causative agent of the emaciation disease like E. leei, but does not transmit from fish to fish. The latter causes no diseases because its pathogenicity is low. Considering these things, it is practically important to identify the parasite species. These two parasites can be differentiated in the presence of the spherical polar capsules for L. fugu and the 2 vegetative nuclei in a primary cell for E. fugu. The sensitive PCR method was developed to detect these parasites. Moreover, non-lethal diagnosis can be performed by samples obtained using a sterile swab inserting into the anal orifice of fish (Yanagida et al., 2005). |
| Other information | Outbreaks of this disease usually occur in summer to late autumn and cease by the end of winter. This may be because the development of E. leei is suppressed by low water temperature (< 15 C.) (Yanagida et al., 2006). There are no effective methods to treat this disease. It is recommended to remove infected fish before introduction of fish by detecting the parasite using the PCR. |
| References | Diamant, A. (1997): Fish-to-fish transmission of a marine myxosporean.
Dis. Aquat. Org., 30,
99-105. Padros, F., O. Palenzuela, C. Hispano, O. Tosas, S. Zahara, S. Crespo and P. Alvarez-pellitero (2001): Myxidium leei (Myxozoa) infections in aquarium-reared Mediterranean fish species. Dis. Aquat. Org., 47, 57-62. Yanagida, T., M. A. Freeman, Y. Nomura, I. Takami, Y. Sugihara, H. Yokoyama and K. Ogawa (2005): Development of a PCR-based method for the detection of enteric myxozoans causing the emaciation disease of cultured tiger puffer. Fish Pathol., 40, 23-28. Yanagida. T., M. Samashima, H. Nasu, H. Yokoyama and K. Ogawa. (2006): Temperature effects on the development of Enteromyxum spp. (Myxozoa) in experimentally infected tiger puffer, Takifugu rubripes (Temminck & Schlegel). J. Fish Dis., 29, 561-567. Yasuda, H., Y. Ooyama, K. Iwata, O. Palenzuela and H. Yokoyama (2002): Fish-to-fish transmission of Myxidium spp. (Myxozoa) in cultured tiger puffer suffering from emaciation disease. Fish Pathol., 37, 29-33. |

(Photos by T. Yanagida)
Fig. 6. A trophozoite (left) and two spores (right) ofEnteromyxum fugu. Note two vegetative nuclei (pink in color) in the primary cell of trophozoite.
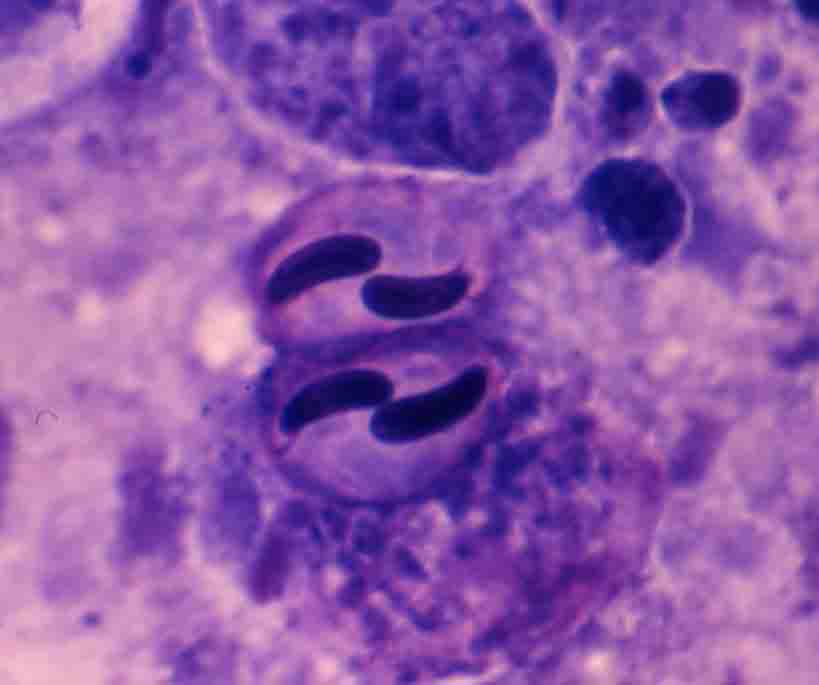
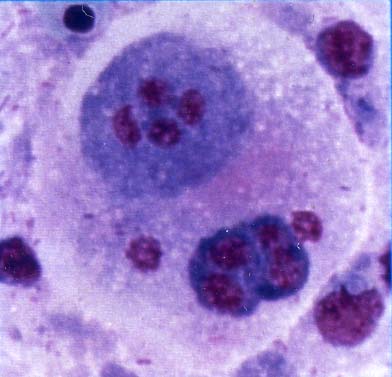
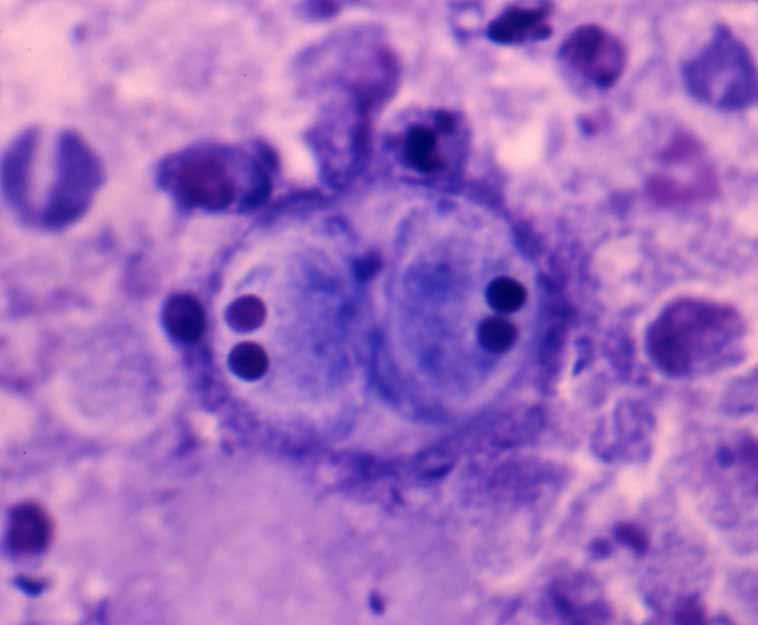
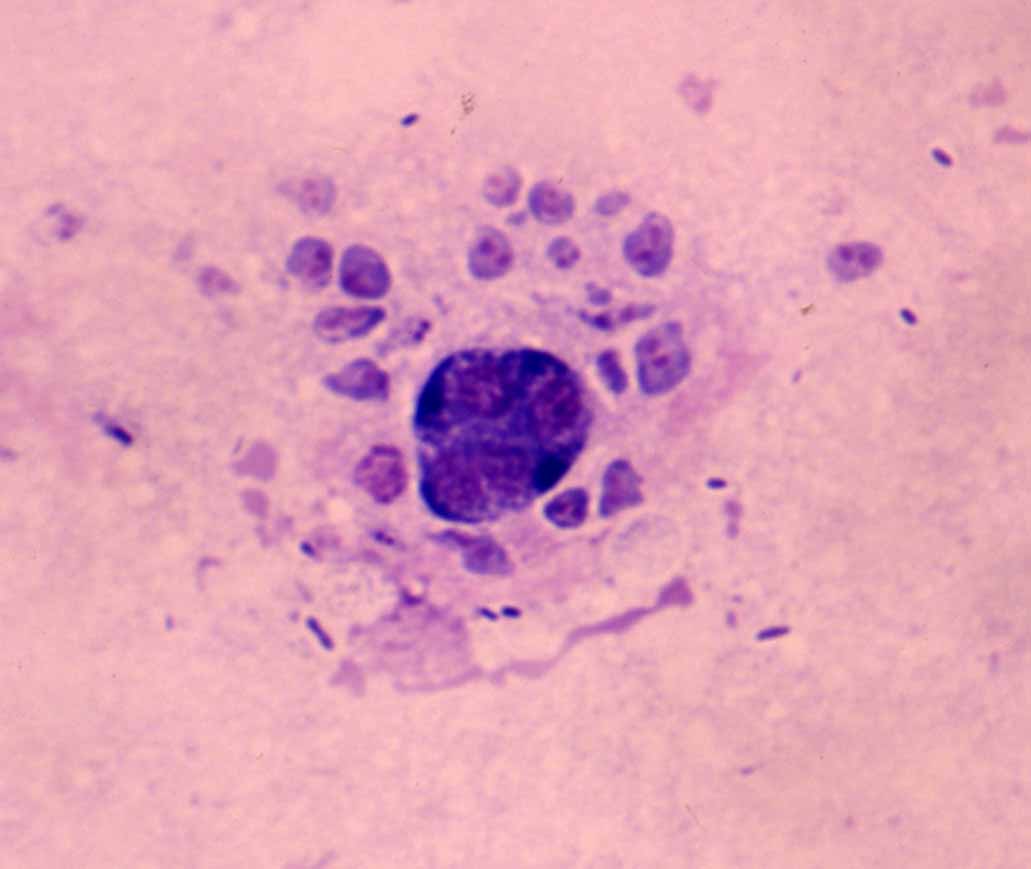
Fig. 3. Mature spore of E. leei from spotted knifejaw.
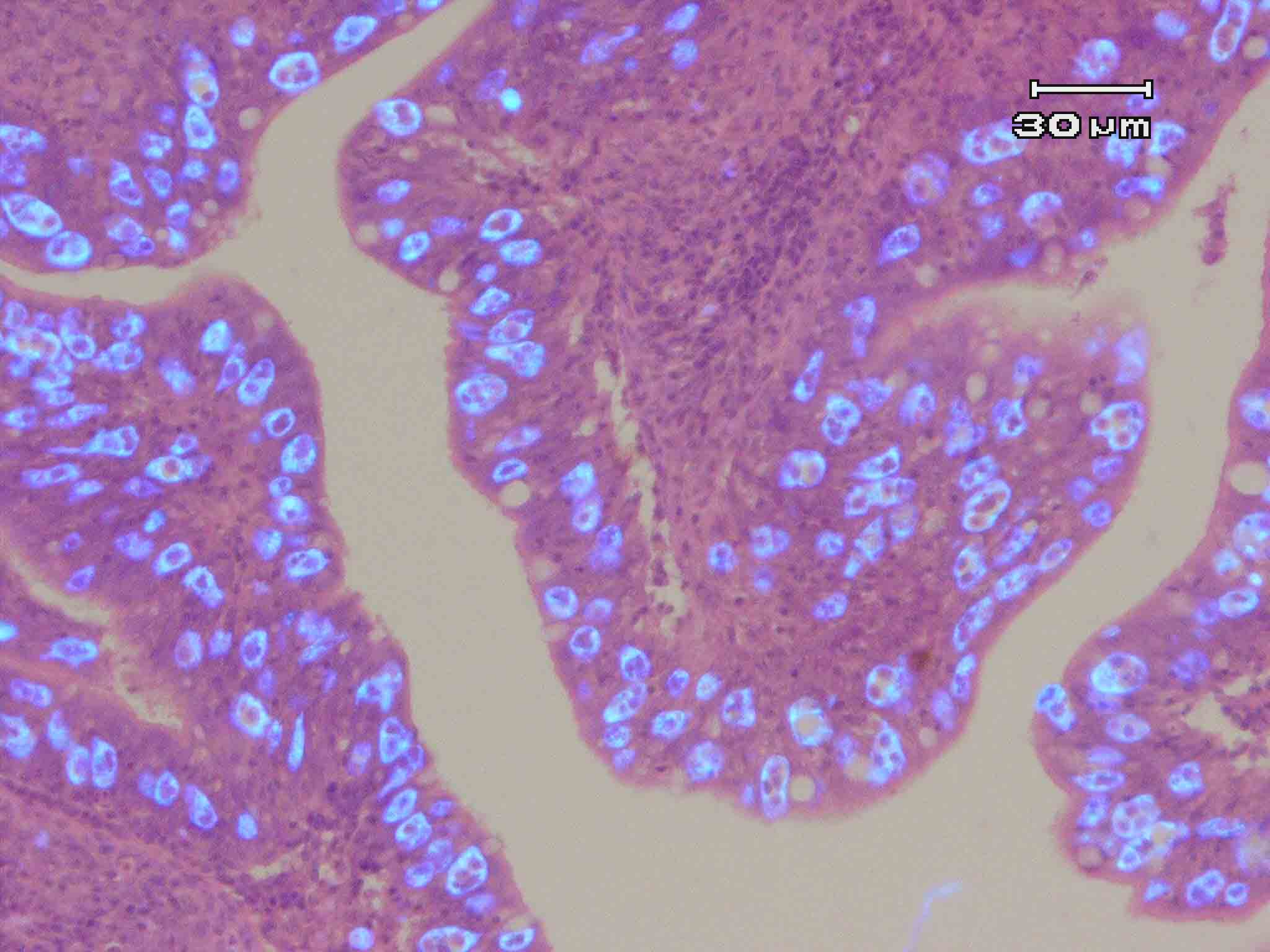
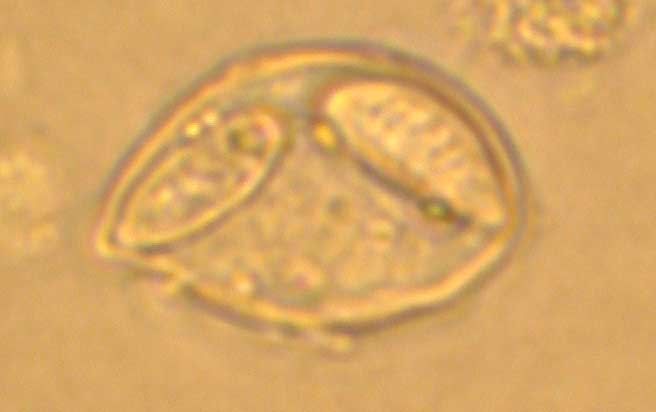
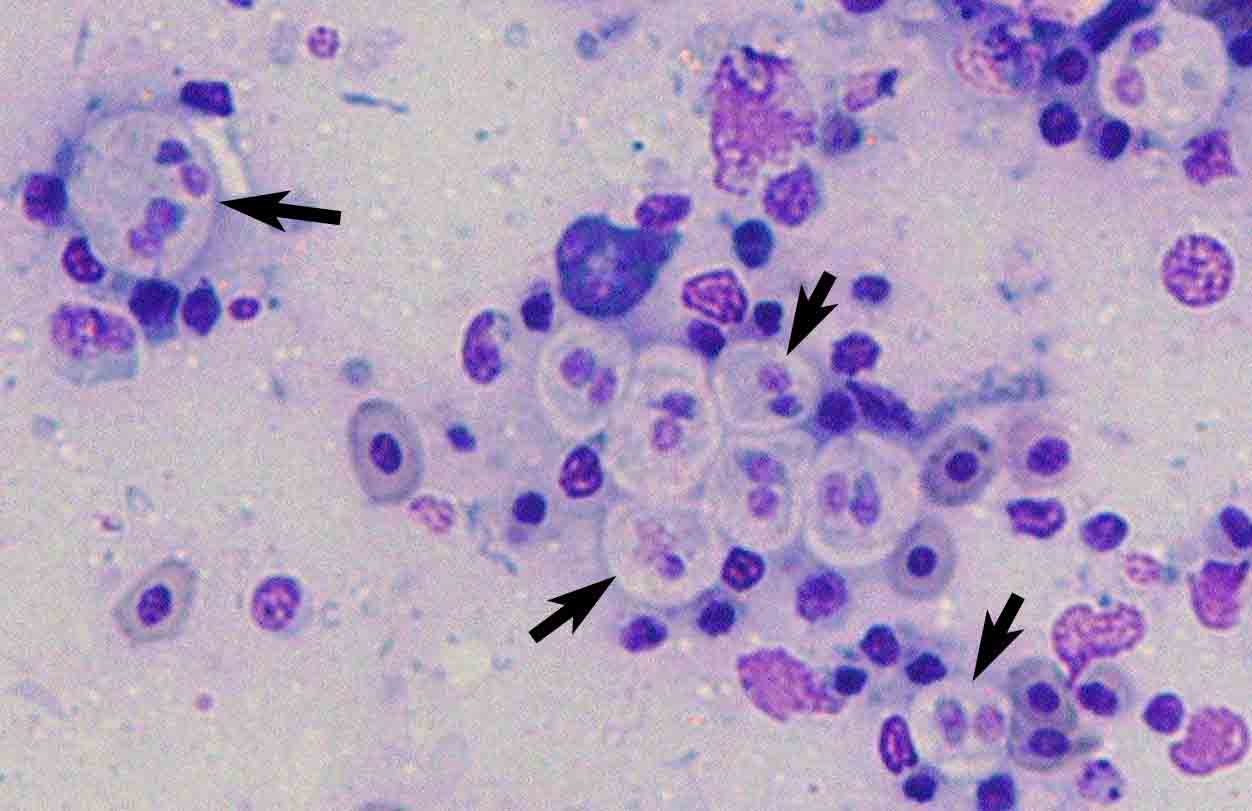
Fig. 5. A trophozoite (left) and two spores (right) of Leptotheca fugu.
Fig. 4. The intestinal epithelia of red sea bream. Uvitex 2B & HE stain.Trophozoites of E. leei emit the fluoresence by Uvitex 2B stain.
Fig. 2. Trophozoites of E. leei (arrows) in a stamp preparation of the intestinal mucosa of tiger puffer. Diff-Quik stain.
Fig. 1. Tiger puffer showing myxosporean emaciation disease.