| Parasite | Kudoa thyrsites |
|---|---|
| Taxonomy | Myxozoa, Myxosporea, Multivalvulida |
| Hosts | Japanese flounder (Paralichthys olivaceus), Splendid alfonsino (Beryx splendens), Japanese anchovy (Engraulis japonicus), Alaska pollock (Theragra chalcogramma), Common dolphinfish (Coryphaena hippurus), Atlantic salmon (Salmo salar), Atlantic mackerel (Scomber scombrus), Shallow-water Cape hake (Merluccius capensis), North Pacific hake (M. productus), John dory (Zeus faber), Dover sole (Microstomus pacificus), Pacific halibut (Hippoglossus stenolepis), Snoek (Thyrsites atun) and over 10 species. |
| Disease name | Muscular kudoosis; Post-mortem myoliquefaction |
| Infection site | Trunk muscle |
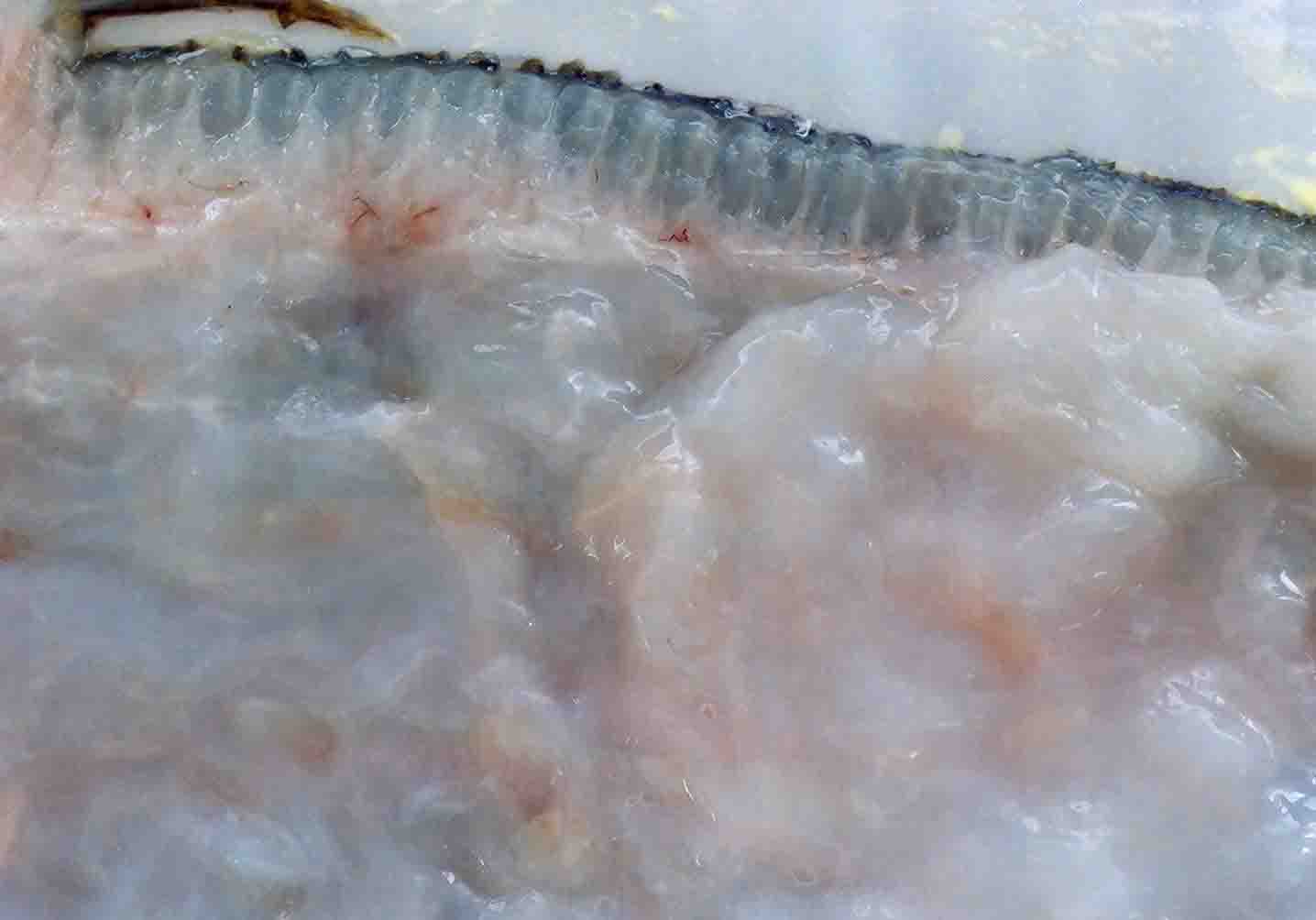
| Clinical sign | The muscle tissue is liquefied after the catch (Fig. 1: Japanese flounder; Fig. 2: Atlantic mackerel). In the early stage of infection or light infection, the diseased fish exhibits the multifocal white foci in the trunk muscle (Fig. 3: splendid alfonsino). Infection is not fatal to host fish. |
| Parasitology | Many spores are produced inside the muscle fibers (Fig. 4). A spore (length 6.9-8.9 Êm; thickness 12.9-8.9 Êm in Japanese flounder) (the spore size differs among the host species) is stellate and has 4 polar capsules, one (length 4.0-5.9 Êm) larger than the other three (length 2.0-4.0 Êm). The life cycle is unknown. |
| Pathology | Host reactions are not observed in the proliferating vegetative stages and no affected area is visible in the infected living fish. After fish death, the myofibrils are disintegrated and the neighboring tissues are liquefied by the proteolytic enzymes derived from the parasite. |
| Health hazard | Since this parasite is not infectious to human, it is harmless in food hygiene. There is no report that the liquefied fish meat is toxic. |
| Diagnosis | Check the spores by wet-mount of the squashed muscle tissue. When the myoliquefactions progress, detection of the spores is sometimes difficult. Kudoa thyrsites is unique because of the different sized polar capsules and distinguished from Kudoa lateolabracis, which also has unequal polar capsules, by the spore size (Yokoyama et al.C2004). Sample should be smeared and stained by Giemsa or Diff-Quik. The detection method by PCR was developed (Hervio et al., 1997). |
| Other information | This parasite is the most studied species in the genus Kudoa because of the wide geographical distribution in the world and the broad host range (Moran et al., 1999; Whipps & Kent, 2006). Atlantic salmon culture in the west coast of Canada is extensively damaged by this disease. On the other hand, the disease occurs sporadically in wild fishes in Japan. Though the Japanese flounder introduced from Korea were suffered from this disease, the source of infection was not clear because this parasite is distributed in Japan (Yokoyama et al., 2004). There are no effective methods to prevent this disease. |
| References | Hervio, D. M. L., M. L. Kent, J. Khattra, J.
Sakanari, H. Yokoyama and R. Devlin (1997): Taxonomy of Kudoa species (Myxosporea), using a small-subunit ribosomal DNA. Can. J. Zool., 75, 2112-2119. Moran J. D. W., D. J. Whitaker and M. L. Kent (1999): A review of the myxosporean genus Kudoa Meglitsch, 1947, and its impact on the international aquaculture industry and commercial fisheries. Aquaculture, 172, 163-196 Whipps, C. M. and M. L. Kent (2006): Phylogeography of the cosmopolitan marine parasite Kudoa thyrsites (Myxozoa: Myxosporea). J. Eukaryot. Microbiol., 53, 364-373. Yokoyama, H., C. M. Whipps, M. L. Kent, K. Mizuno and H. Kawakami (2004): Kudoa thyrsites from Japanese flounder and Kudoa lateolabracis n. sp. from Chinese sea bass: causative myxozoans of post-mortem myoliquefaction. Fish Pathol., 39, 79-85. |
Fig. 4. Fresh spores of K. thyrsites

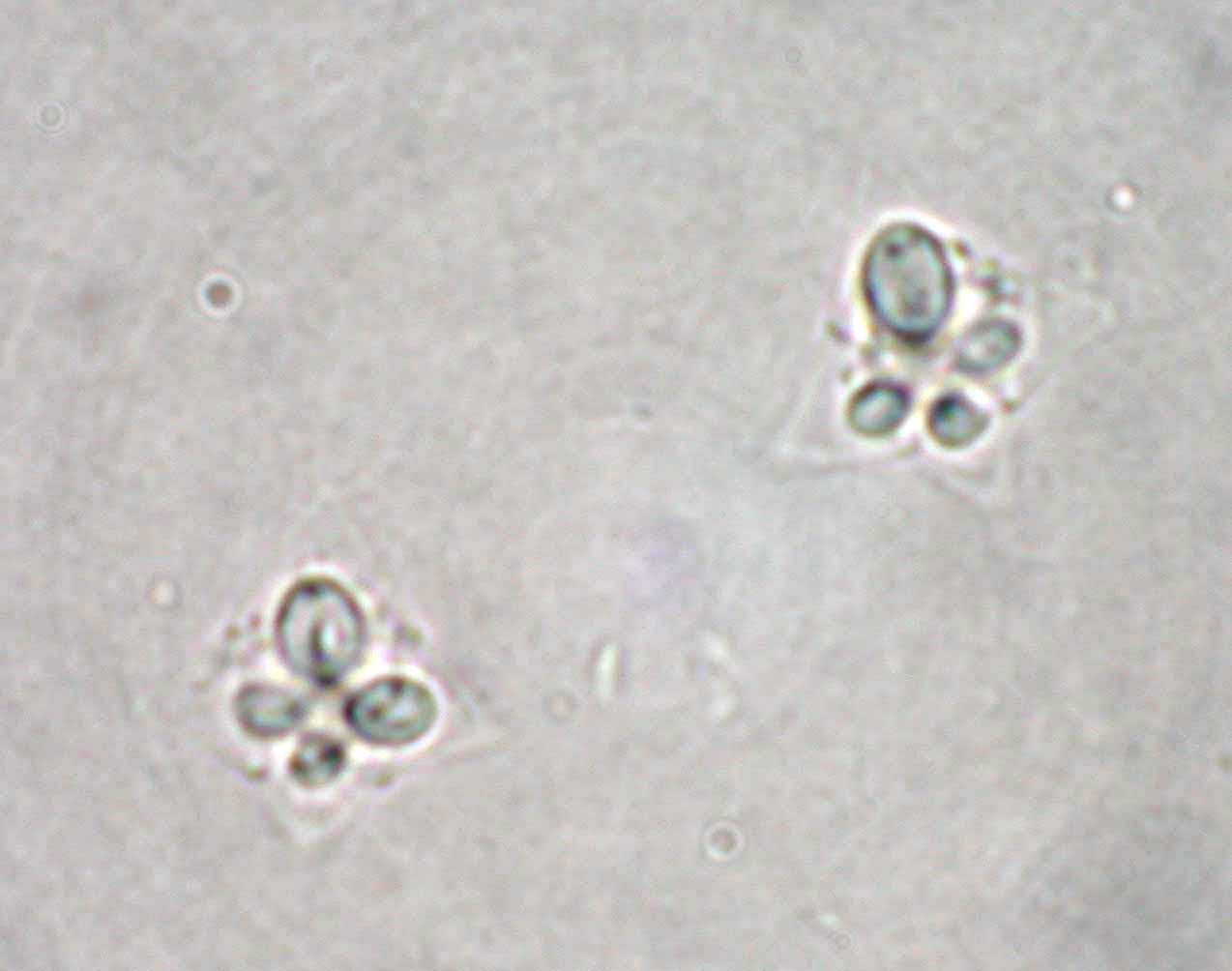
Fig. 3. Fillet of splendid alfonso showing multiple foci of Kudoa.
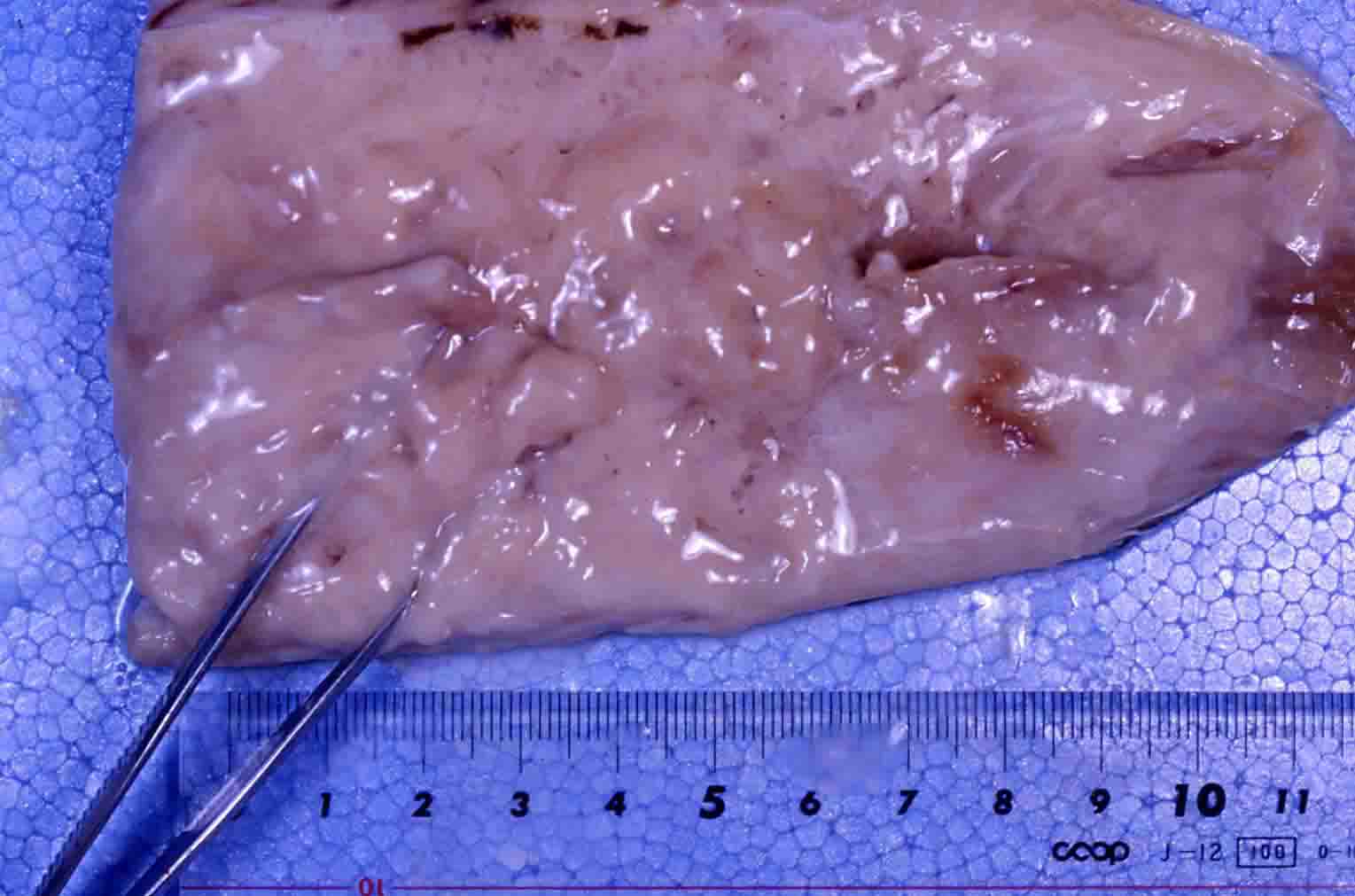
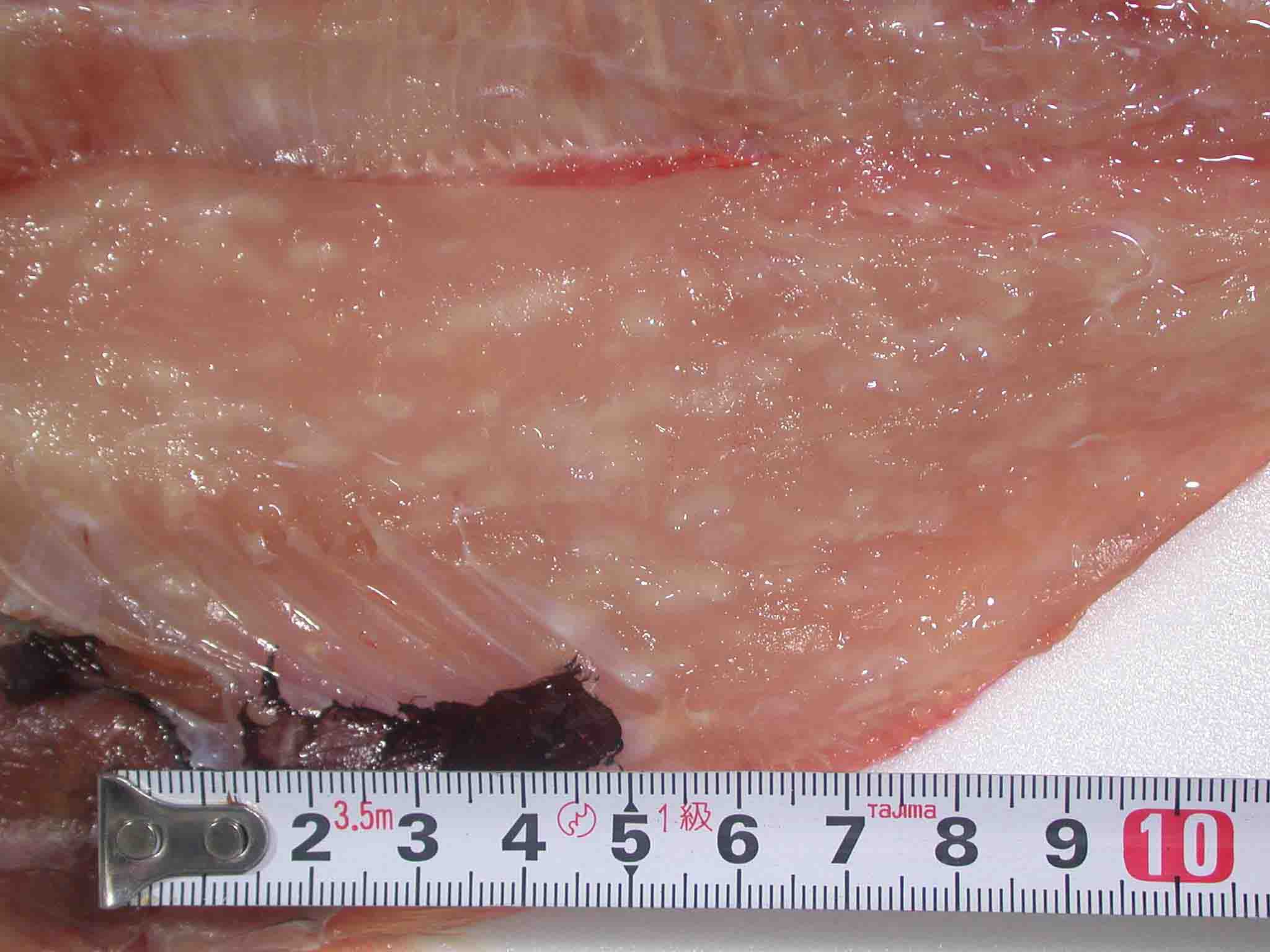
Fig. 1. Fillet of Japanese flounder showing the liquefaction.
Fig. 2. Fillet of Atlantic mackerel showing a liquefaction.