
(Photos by S. Urawa (2) and S. Miyajima (1, 3))
| Parasite | Kabatana takedai |
|---|---|
| Taxonomy | Microspora, Microsporea |
| Hosts | Masu salmon (Oncorhynchus masou), pink salmon (O. gorbuscha), chum salmon (O. keta), chinook salmon (O. tshawytscha), sockeye salmon (O. nerka), rainbow trout (O. mykiss), whitespotted char (Salvelinus leucomaenis), dolly varden (S. malma), broun trout (Salmo trutta) |
| Disease name | Microsporidiosis takedai |
| Infection site | Skeletal muscle, heart muscle, a part of smooth muscle |
| Clinical signs | No external signs are observed in light infection, while depressed body and enlarged heart are evident in heavy infection. White cysts (aggregates of hostfs tissues and spores) are observed in the trunk muscle and the heart muscle. A cyst is max. 6mm long and spindle-shaped (Fig. 1). Over 200 cysts are seen in heavily infected fish. |
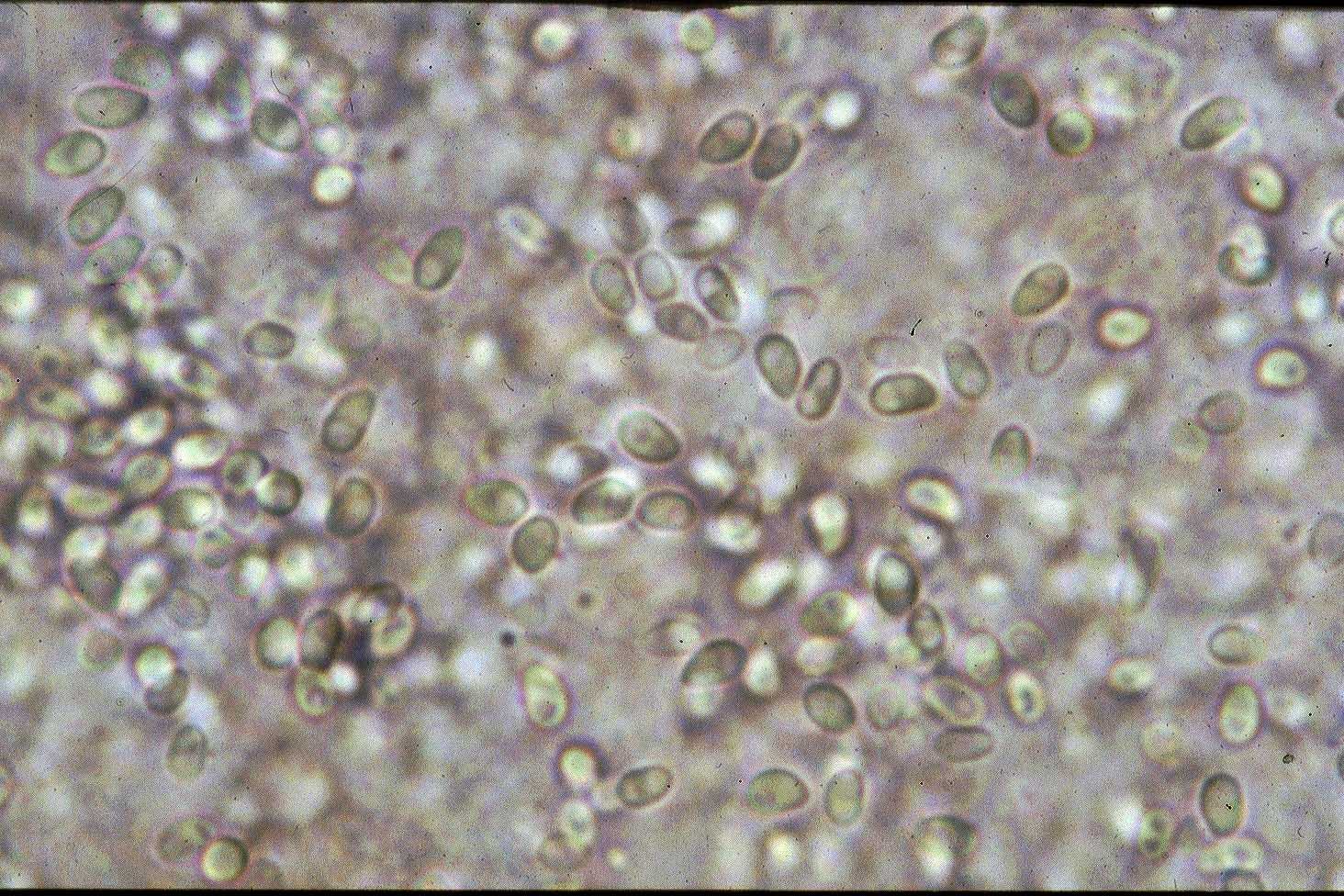
| Parasitology | Kabatana takedai invades the muscle tissue of a host, and proliferates followed by sporulation. A spore (length 2.8-4.9 Κm; width 1.7-2.3 Κm) is oval (Fig. 2). Development of the parasite was influenced by the water temperature; inhibited at 11 C., activated at 13-17 C. However, proliferation of parasite started when the water temperature was raised at 18 C. (Zenke et al., 2005). The life cycle is unknown. This parasite doesnft transfer from fish to fish (Fujiyama et al., 2002). |
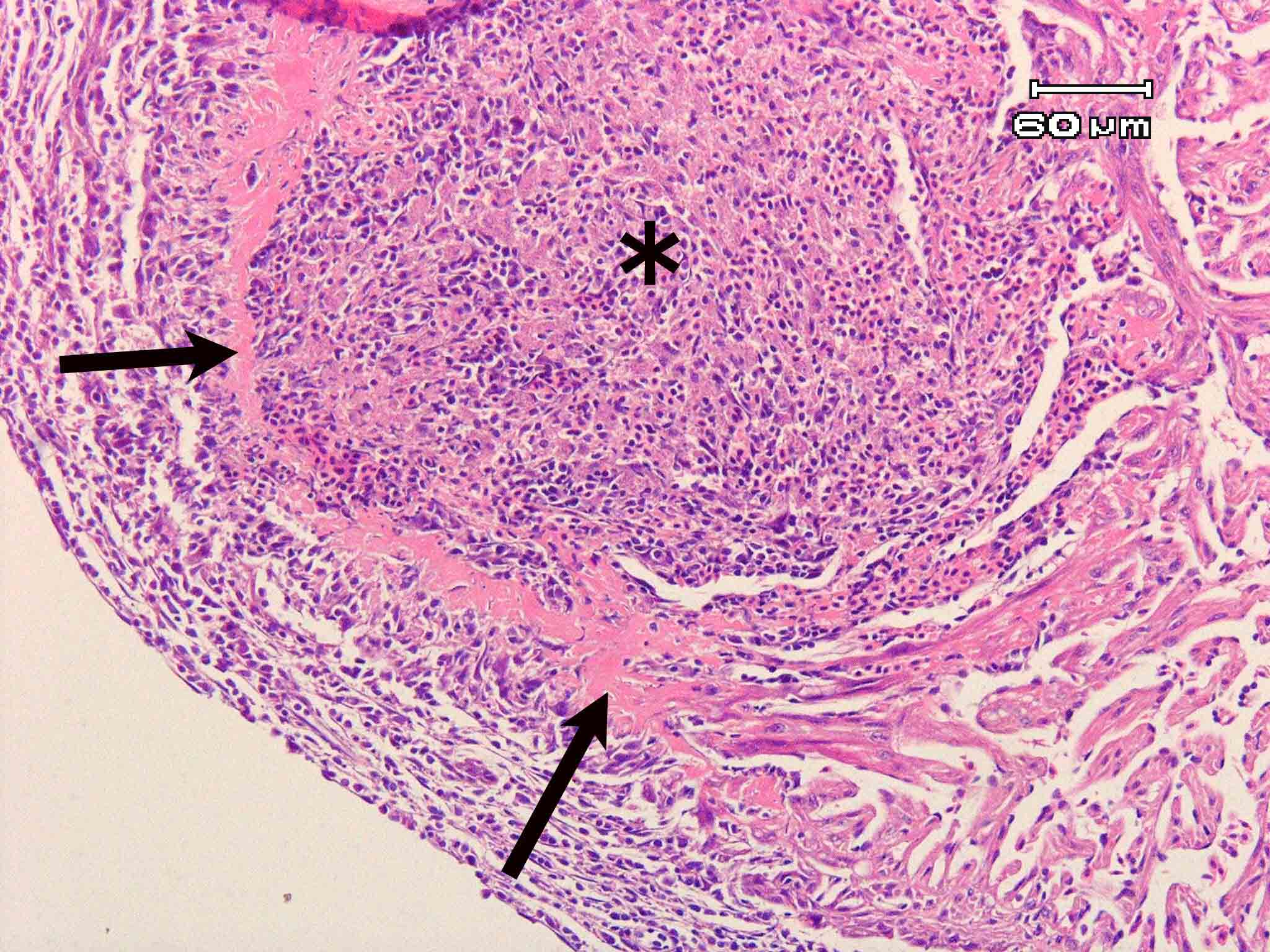
| Pathology | In the early phase of infection, no host reactions are observed around a mass of parasite developing in the endocardium. Eventually, foci become granulomatous and spores are phagocytized by macrophages following to infiltration of the inflammatory cells, degeneration of the myofibrils and proliferation of the connective tissue (Fig. 3). Fibrinoid degeneration occurs in the marginal area of foci. In the trunk muscle, infiltration of the inflammatory cells and degeneration and rupture of the myofibrils occur, but extensive proliferation of the connective tissue is not observed (Awakura, 1974; Miyajima et al., 2007). It is considered that infected fish is led to the death since the fish becomes sensitive to the lack of oxygen (Urawa, 2006). |
| Health hazard | Since this parasite is not infectious to human, it is harmless in food hygiene. |
| Diagnosis | Check the spores by the wet-mount of cysts. The sample should be smeared and stained by Uvitex 2B followed by a fluorescent microscopic observation. The stained spores emit blue fluorescence under UV radiation (Fig. 4). The detection method by PCR was developed (Miyajima et al., 2007). |
| Other information | Kabatana takedai is known as an enzootic pathogen which has been reported from the limited different water systems including the Chitose River, Lake Akan Lake Tokito-numa in Hokkaido and the Taranay River and the Bryanka River in Sakhalin. This disease occurs in salmonid fishes reared by the Chitose River water in summer to autumn. Approximately 100,000 masu salmon fry were slaughtered to prevent the parasite spreading (Urawa, 1989). Occurrence of the disease can be controlled by keeping the low water temperature, but this method is not practical since fish growth is also retarded by the low temperature. |
| References | Awakura, T. (1974):
Studies on the microsporidian infection in salmonid fishes. Sci. Rep. Hokkaido Fish Hatchery, 29, 1-95. Fujiyama, I., S. Urawa, H. Yokoyama and K. Ogawa (2002): Investigation of the transmission stage of the microsporidian Kabatana takedai in salmonids. Bull. Natl. Salmon Resources Center, 5, 1-6. Miyajima, S., S. Urawa, H. Yokoyama and K. Ogawa (2007): Comparison of susceptibility to Kabatana takedai (Microspora) among salmonid fishes. Fish Pathol., 42, 149-157. Urawa, S. (1989): Seasonal occurrence of Microsporidium takedai (Microsporida) infection in masu salmon, Oncorhynchus masou, from the Chitose River. Physiol. Ecol. Japan, Spec., 1 587-598. Urawa, S. (2006): Microsporidian infection. New atlas of fish diseases (ed. by Hatai, K. and K. Ogawa), Midori Shobo, p. 38. (In Japanese) Zenke, K., S. Urawa, I. Fujiyama, H. Yokoyama and K. Ogawa (2005): Effects of water temperature on infection of the microsporidian Kabatana takedai in salmonids. Fish Pathol., 40, 119-123. |
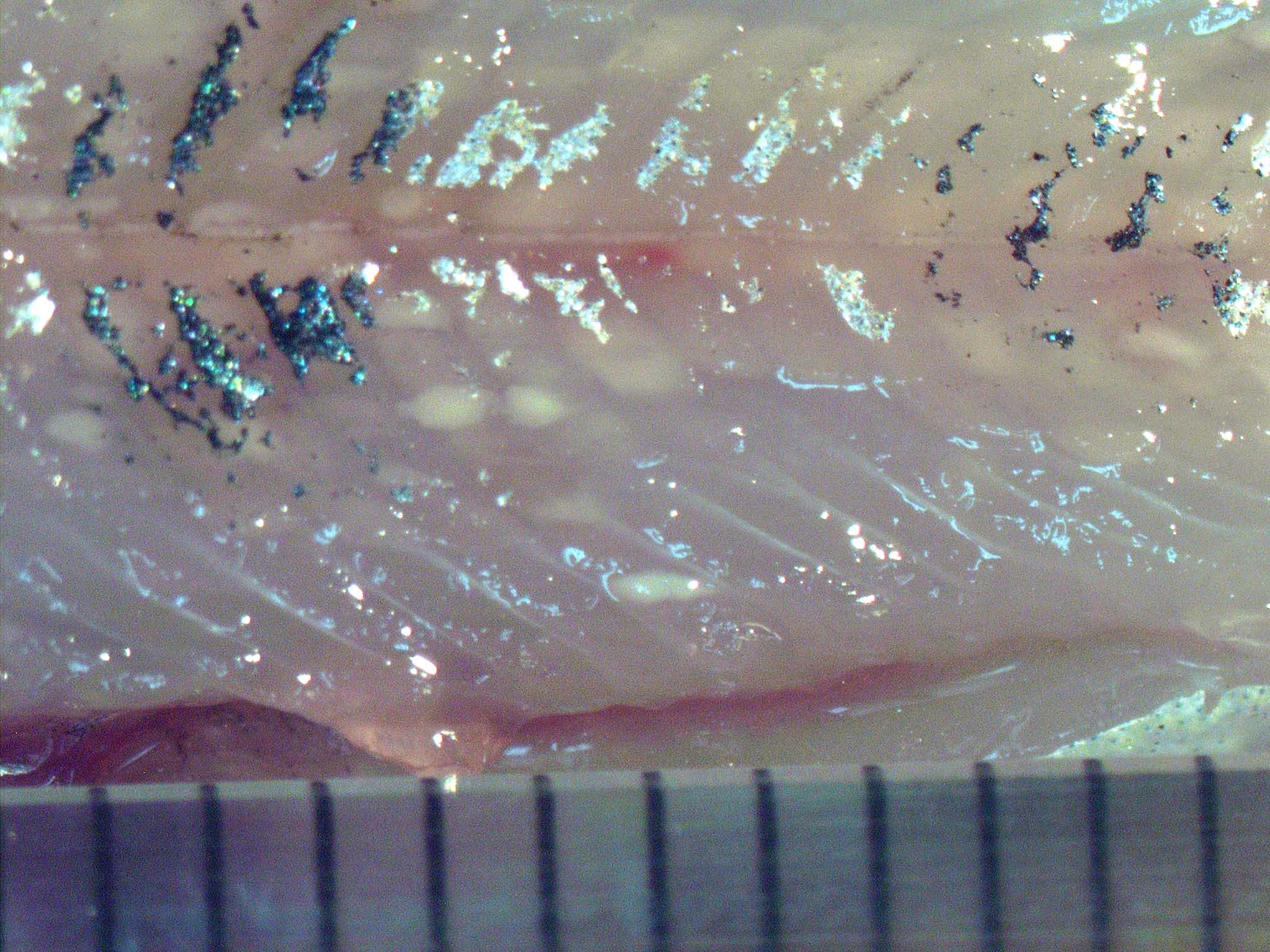
Fig. 3. Fibrinoid degeneration seen as eosinophilic ring (arrows) surrounding the parasite focus (asterisk).
Fig. 1. Numerous foci formed in the trunk muscle of
masu salmon.
Fig. 2. Fresh spores of Kabatana takedai